

The National Council for Mental Wellbeing † supports the safe and effective use of LAIs by encouraging mental healthcare professionals to utilize LAIs as an earlier treatment option.
†Formerly known as the National Council for Behavioral Health.
LAI=long acting injectable.
A randomized, double-blind, placebo-controlled, long-term maintenance study compared 3-month INVEGA TRINZA ® with placebo 2,3
A noninferiority study
compared INVEGA TRINZA ® to INVEGA SUSTENNA ®4
An international, open-label study examined symptomatic remission and goal attainment with INVEGA TRINZA ® 5,6
One Dose of INVEGA TRINZA ® Delivers 3 Months of Sustained Plasma Concentration 2,7
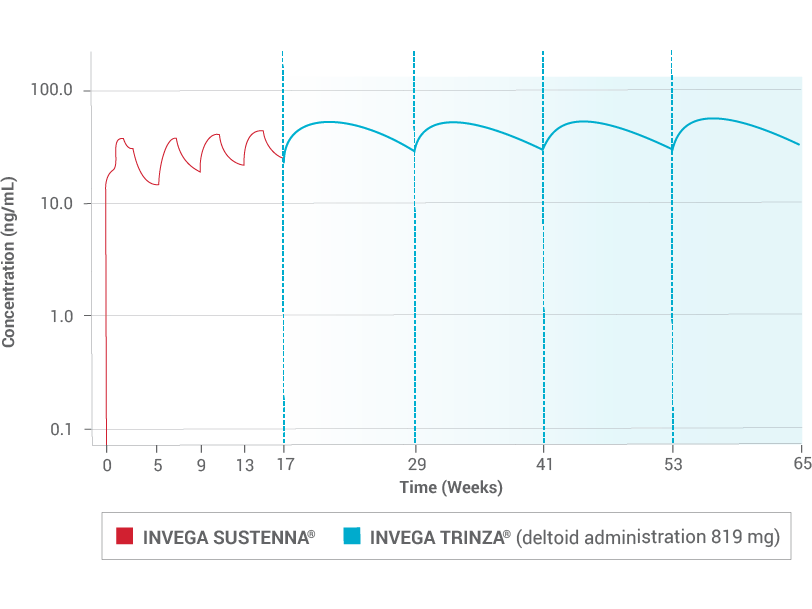
Due to the difference in median pharmacokinetic profiles between the 2 products (INVEGA TRINZA ® and INVEGA SUSTENNA ® ), caution should be exercised when making a direct comparison of their pharmacokinetic properties.
Correlation to clinical effect has not been established.



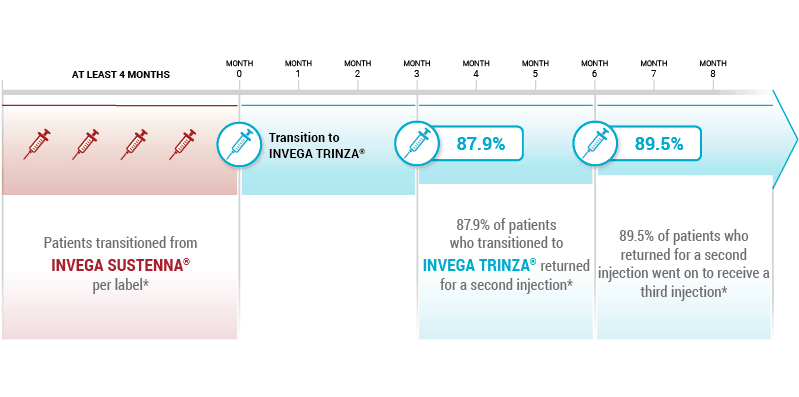
Based on a retrospective cohort analysis of pharmacy and medical claims data, of adult patients with schizophrenia (n=1603), from the Symphony Health Solutions (SHS) database from May 2014 to September 2016. First data on patient claims for INVEGA TRINZA ® were from July 2015.
*Among patients with ≥4 months of follow-up who transitioned from INVEGA SUSTENNA ® to INVEGA TRINZA ® per label (defined as consistent INVEGA SUSTENNA ® coverage for ≥4 months, with the same dosage strength).
Patient claims data
Symphony Health, a robust open-source database, captures prescription information for more than 280 million patients. The database does not capture services received outside of the Symphony Health network. 10